PLATELET-RICH PLASMA
A PLATFORM TO PROCESS AND CAPTURE OPTIMAL PLATELET CONCENTRATES
A WIDELY
RECOGNIZED
REGENERATIVE
TREATMENT
The use of Platelet-Rich Plasma (PRP) has become a widely recognized regenerative treatment for aesthetic patients.
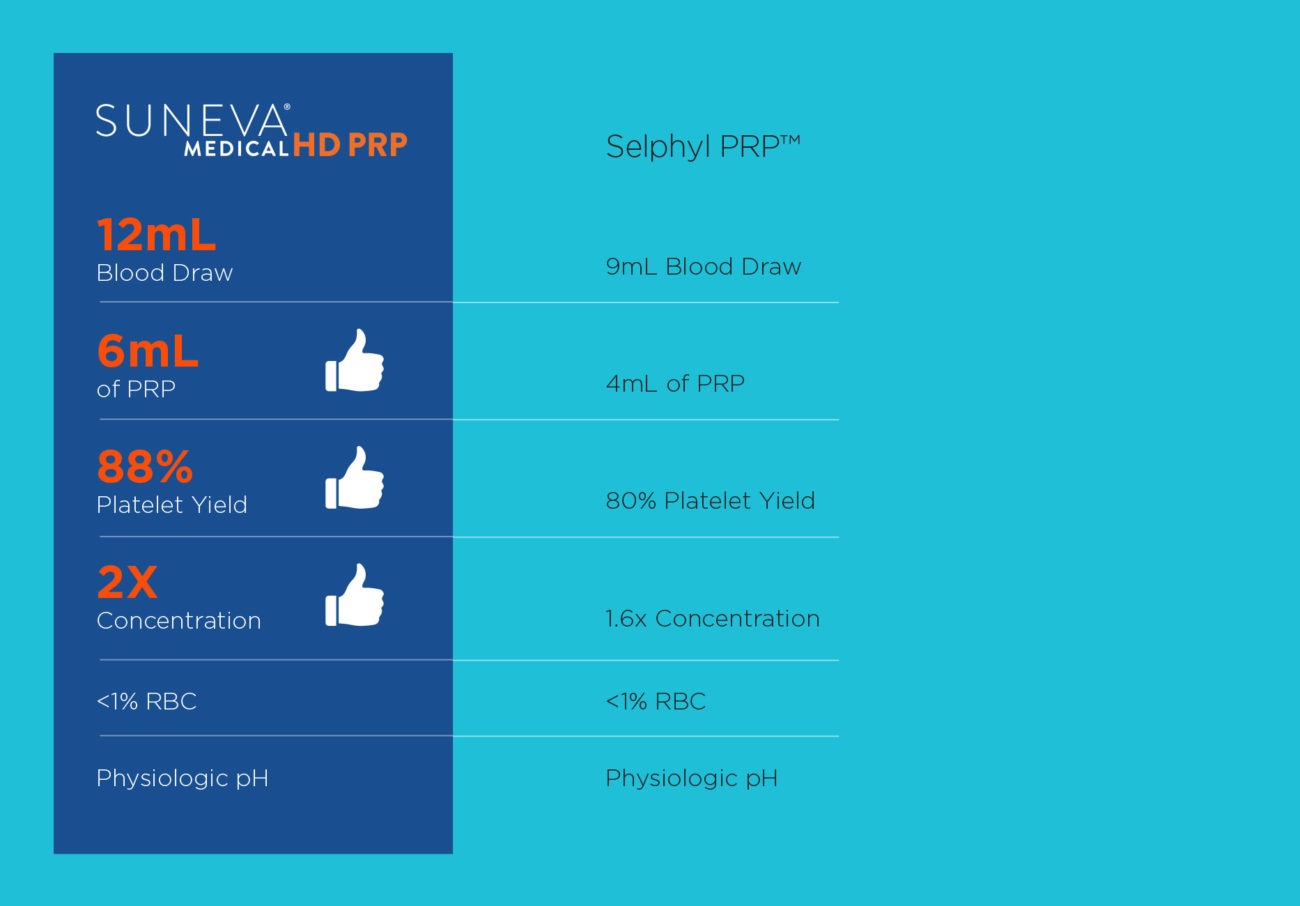
But not all PRP is the same. Higher levels of PRP concentration are required to be effective.

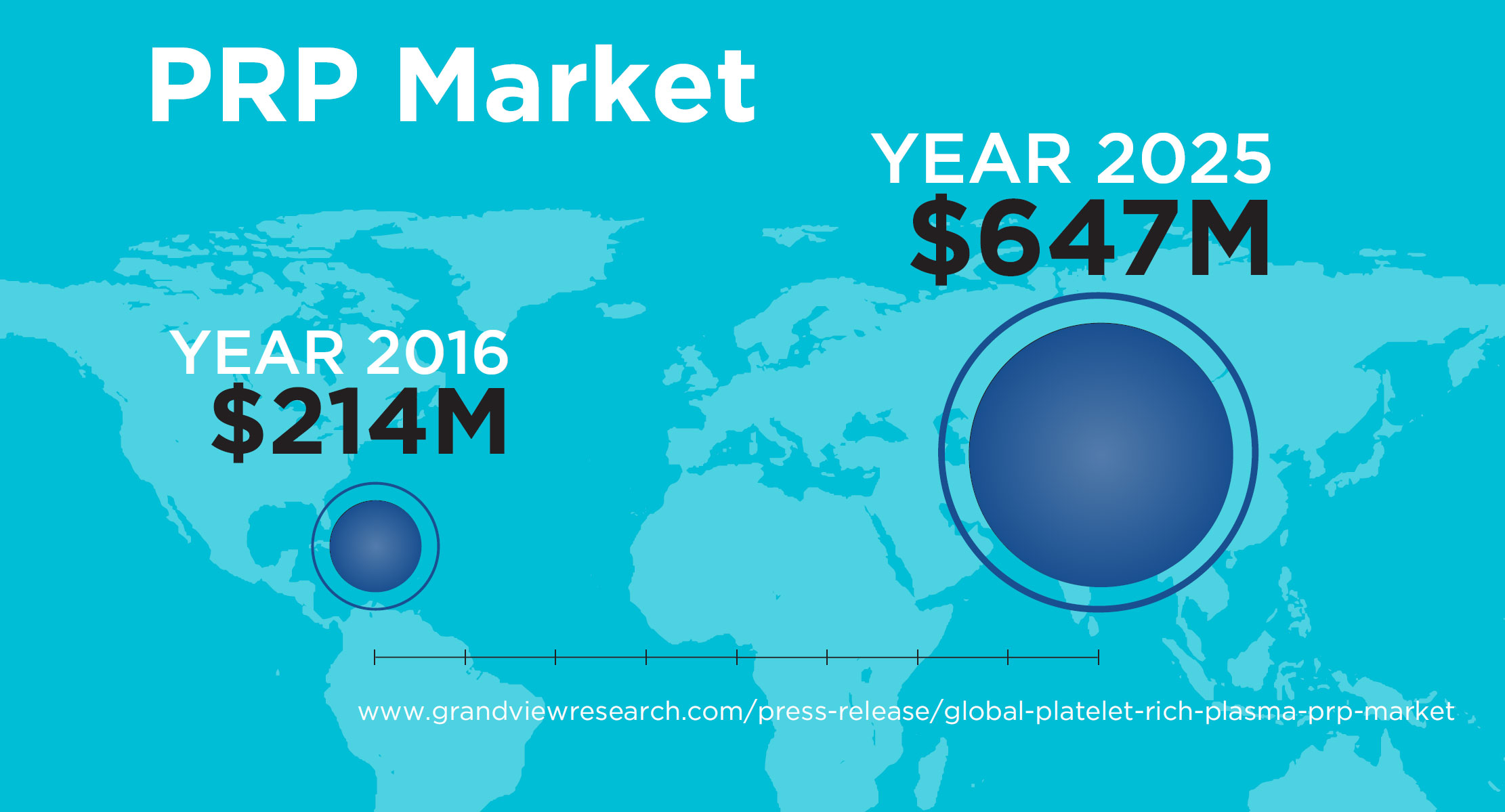
CONSUMER DEMAND
Consumer demand is driving the regenerative aesthetics market as patients seek more natural, minimally invasive solutions to achieve a more youthful appearance.
THE HIGHEST
PLATELET YIELD
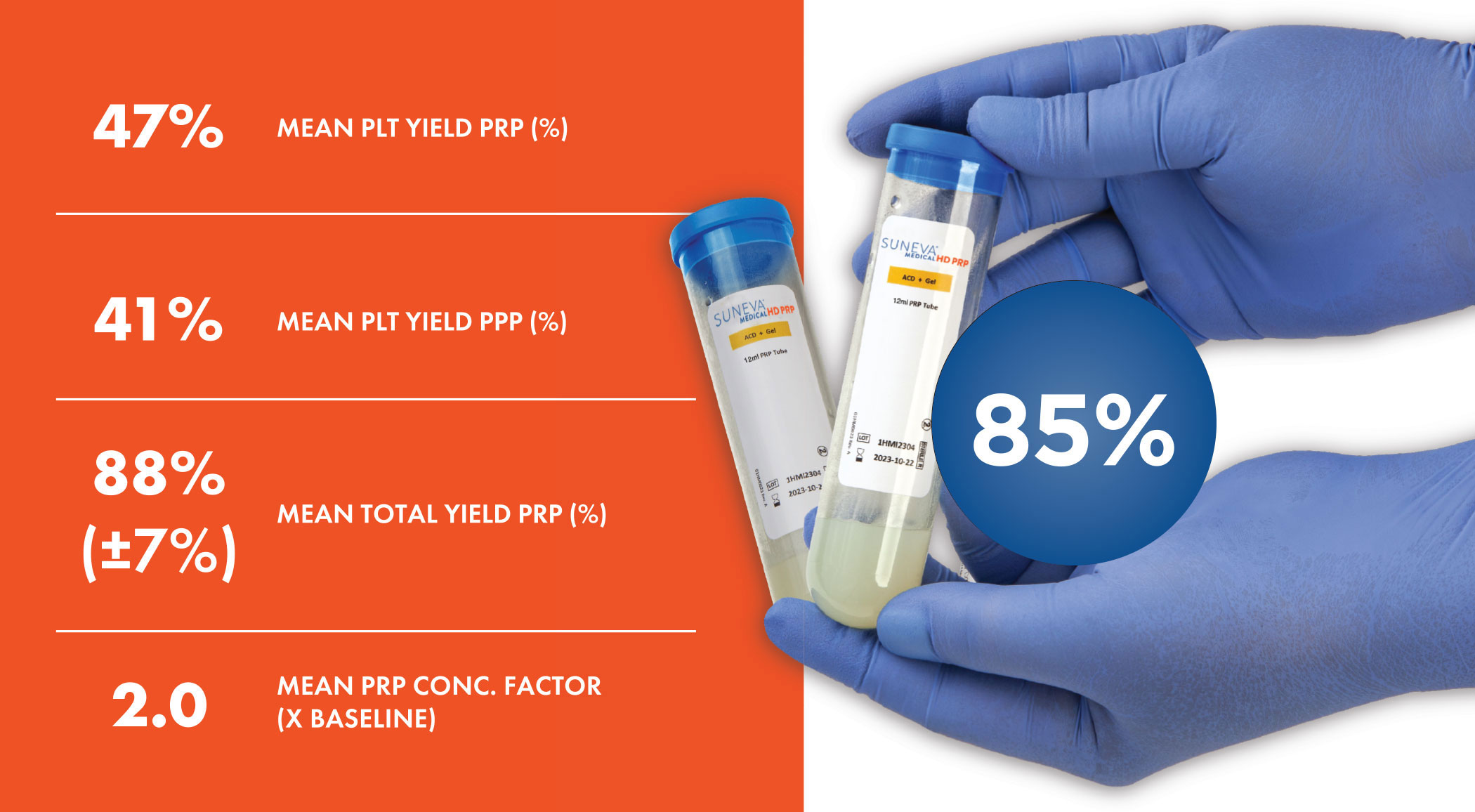
Suneva Medical HD PRP boasts a robust platelet yield of ~88% compared to other gel separator systems.
- Concentration system uses a unique separating gel to greatly improve the collection of PRP
- Yields the most platelets per mL from the smallest blood draw
- Designed to accommodate busy practices
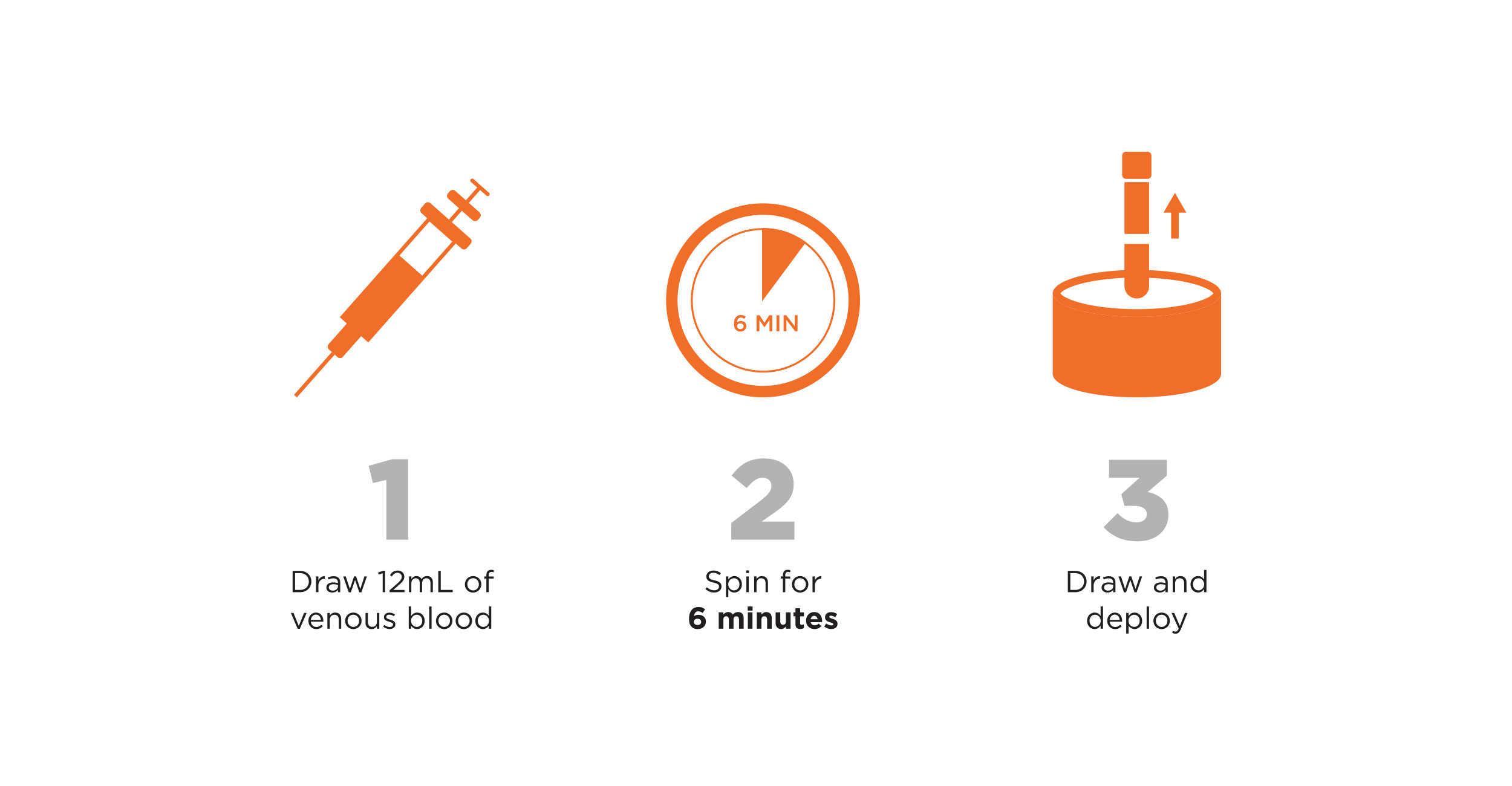
SIMPLE, THREE-STEP PROCESS
Shorter Processing Cycle = Increased Practice Efficiency
Suneva Medical HD PRP is the quickest and most e fficient concentrating system available.
SHORTER PROCESSING CYCLE = INCREASED PRACTICE EFFICIENCY
Suneva Medical HD PRP is the quickest and most efficient concentrating system available.
Designed to accommodate providers that run a busy practice, the entire process takes less than 20 minutes.
PRP is a great standalone or complimentary procedure.