THE FIRST FDA CLASS II CLEARED
HANDHELD PLASMA DEVICE

ENERGY-BASED RENEWAL
Plasma IQ is the latest advancement in aesthetics that delivers focused, controlled plasma energy to safely and effectively create microinjuries on the skin, renewing and restoring it.
Microbeams of plasma are leveraged in a focused energy treatment, resulting in tightening and retraction of the skin tissues through controlled skin damage.
SAFE, QUICK AND EFFECTIVE
> Plasma IQ has a low risk of side effects. Extreme device precision and controlled energy settings limit the damage of surrounding skin, reduce downtime and improve healing.> Plasma IQ treatment can be performed in-office in as little as 30 minutes, depending on the area(s) treated.
> Two levels of energy allow for safe and effective treatments for a variety of indications and areas. Plasma IQ delivers the precise amount of energy desired using the high (950V) or low (650V) settings during treatment.

THE FIRST FDA CLASS II
HANDHELD PLASMA DEVICE
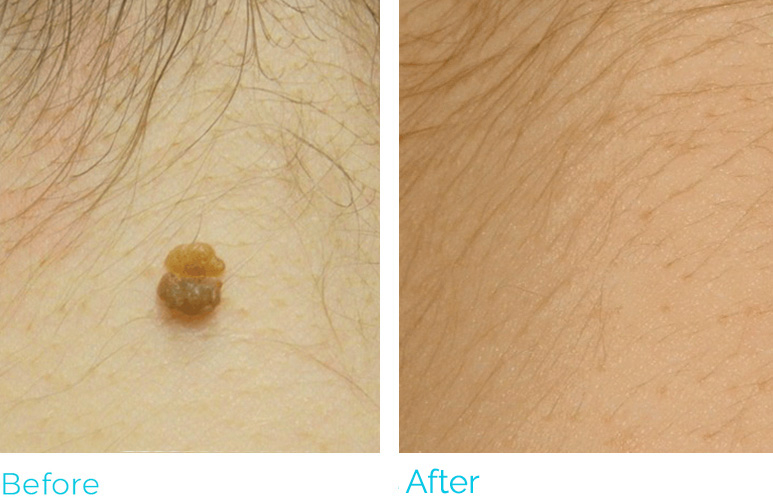
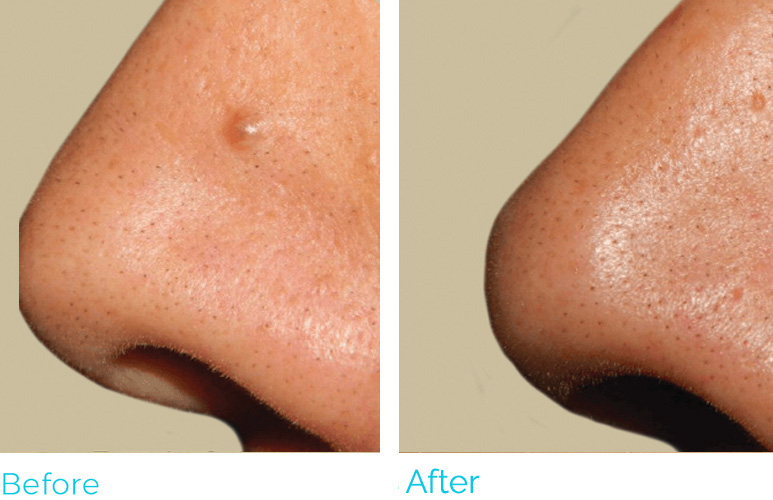
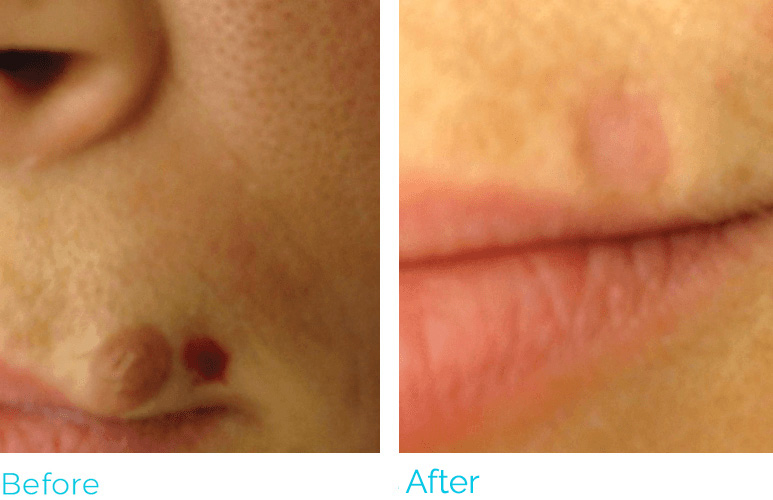
PLASMA IQ IN ACTION
REAL RESULTS
HOW IT WORKS
IONIZED GAS PARTICLES As the device is brought close to the skin during treatment, it ionizes nitrogen gas particles in the air to create a plasma arc that looks like a spark.
SKIN RETRACTION AND REACTION The plasma arc causes sublimation of the epidermis of the skin; turning a solid into gas, resulting in immediate skin tightening and retraction in the target area. This method of plasma sublimation is highly targeted and controlled. It does not transfer heat to the surrounding area.
The procedure creates tiny brown carbon crusts or scabs in the treated area that persist for approximately one week before dropping off.
HEALING RESPONSE The microinjuries sustained during treatment promote the long-term firming, tightening, and smoothing effects that are part of the natural healing response.

CONTACT A SUNEVA REPRESENTATIVE TODAY
PLASMA IQ is FDA cleared to be used in the removal and destruction of skin lesions and the coagulation of tissue. The most common side effects are swelling, tenderness, scabbing and redness. PLASMA IQ is Rx only and should only be used by medically licensed and certified practitioners. For full product and safety information, visit https://www.sunevamedical.com/ifu/ ©2020 Neauvia North America