BIOSTIMULATOR
THE ONLY FDA APPROVED 5-YEAR FILLER THAT STIMULATES PATIENT’S OWN NATURAL COLLAGEN.
THE “MUST-HAVE” PRODUCT FOR YOUR AESTHETIC PRACTICE
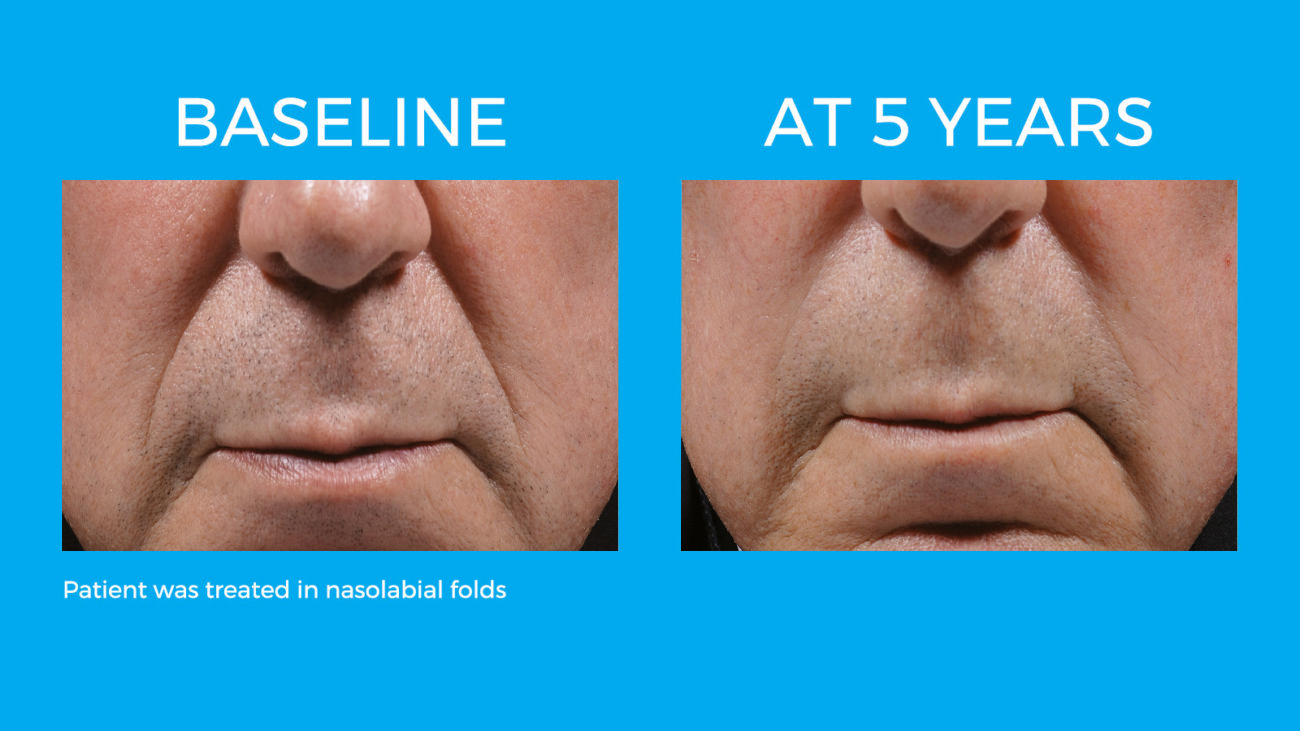
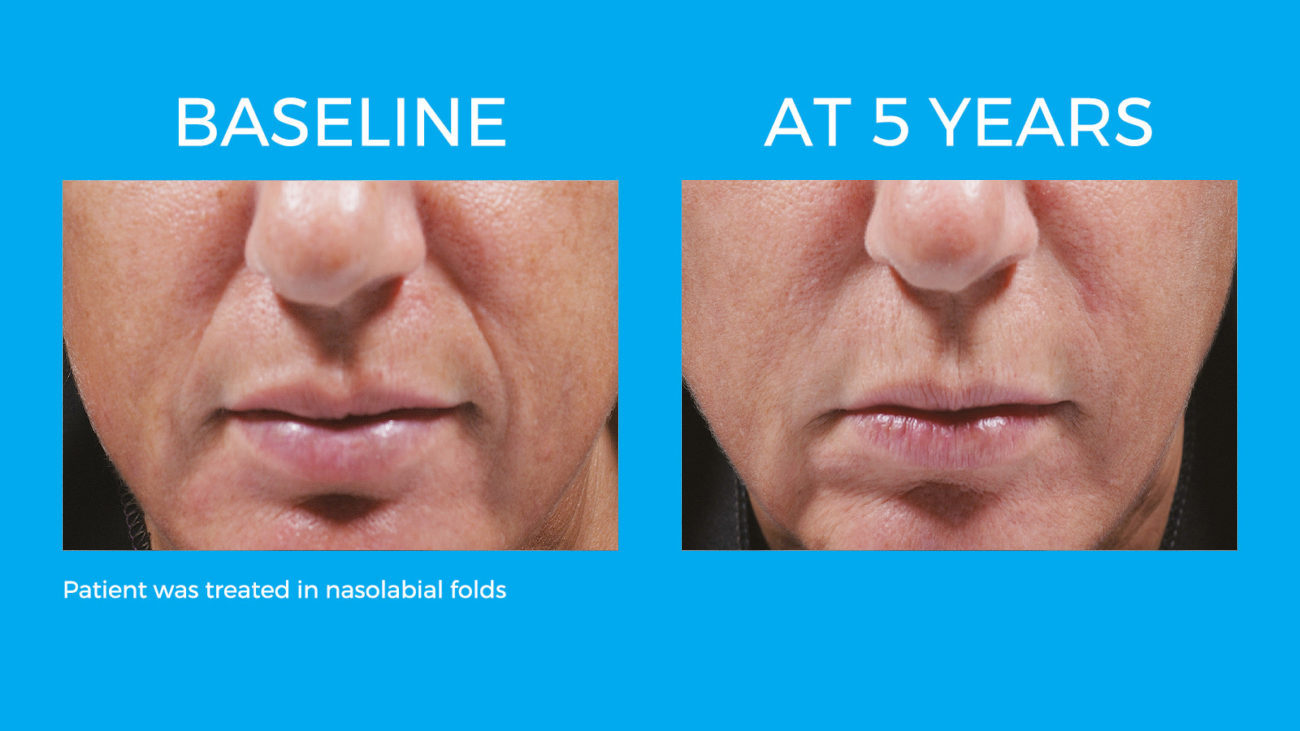
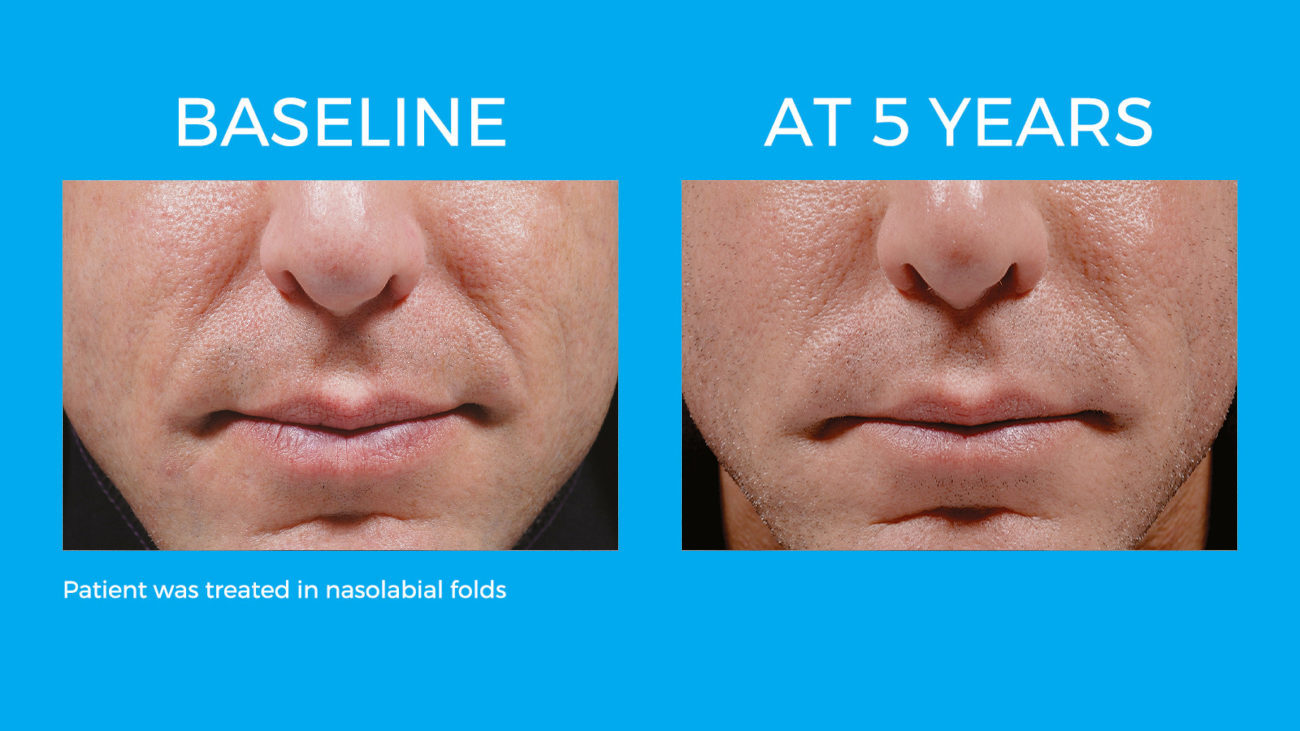
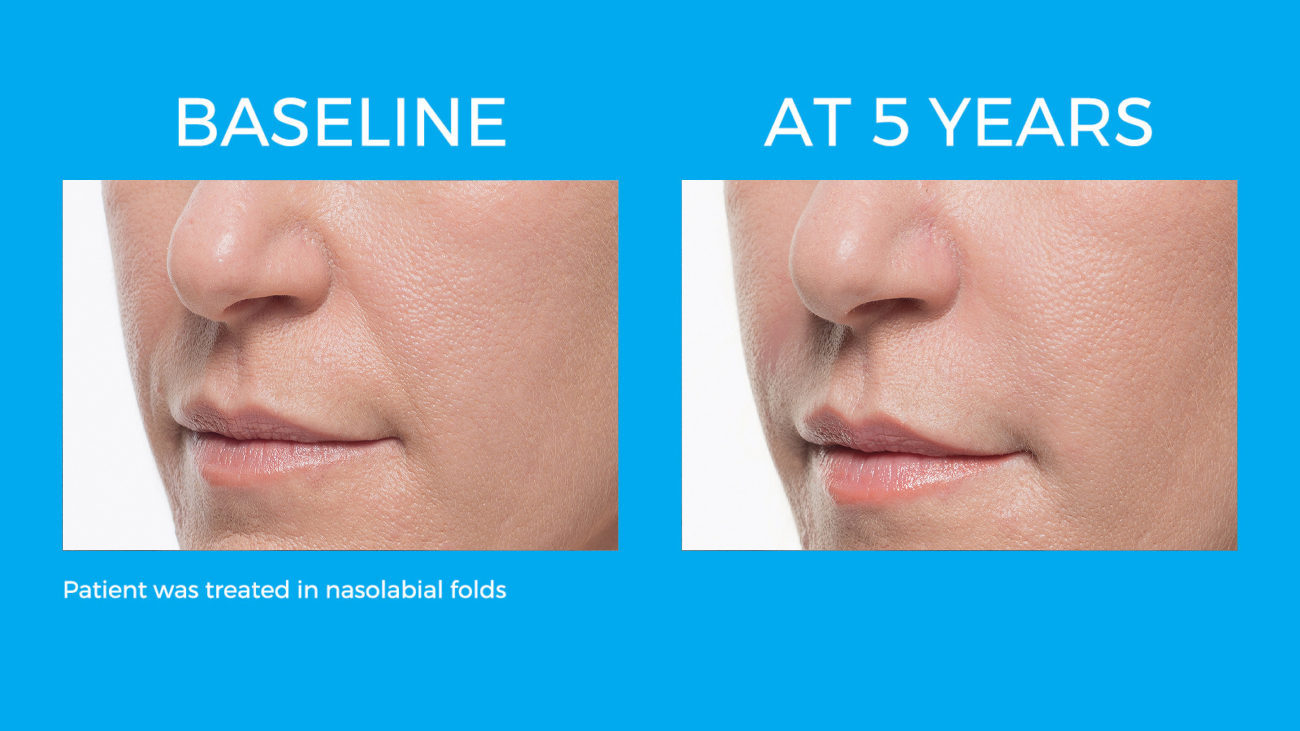
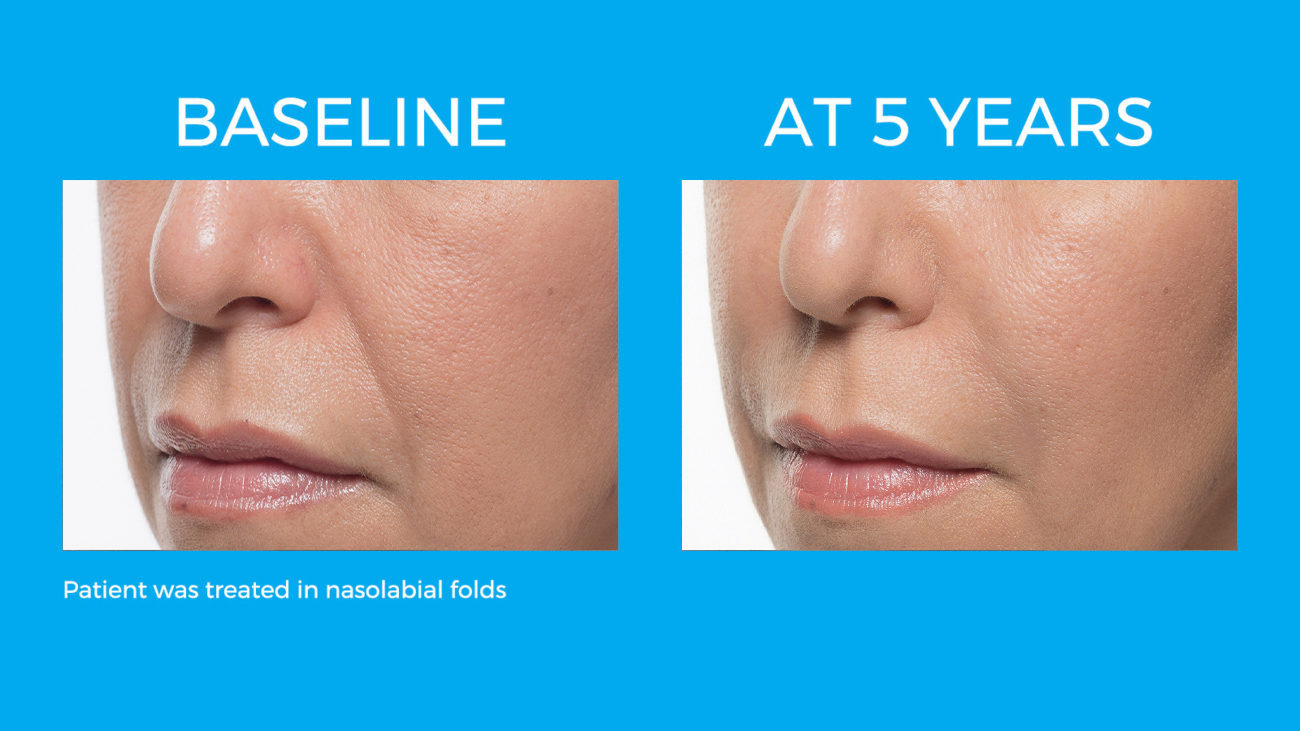
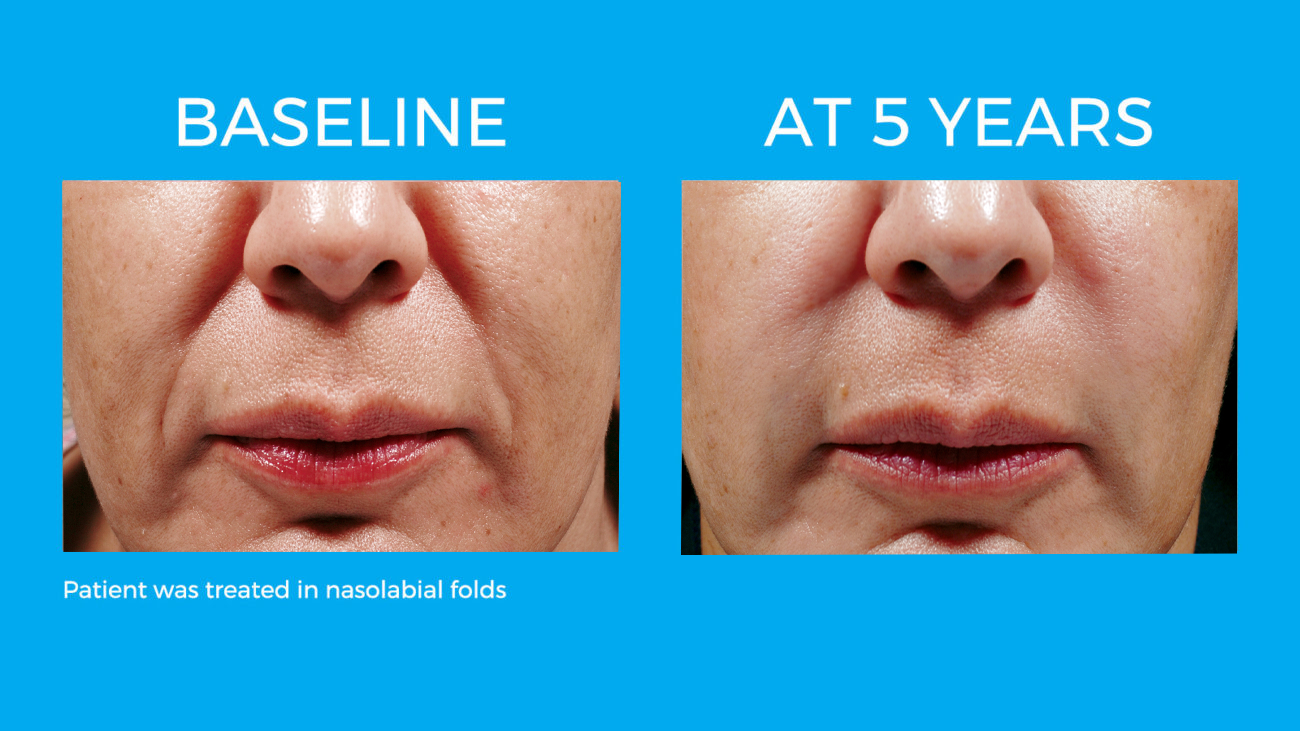
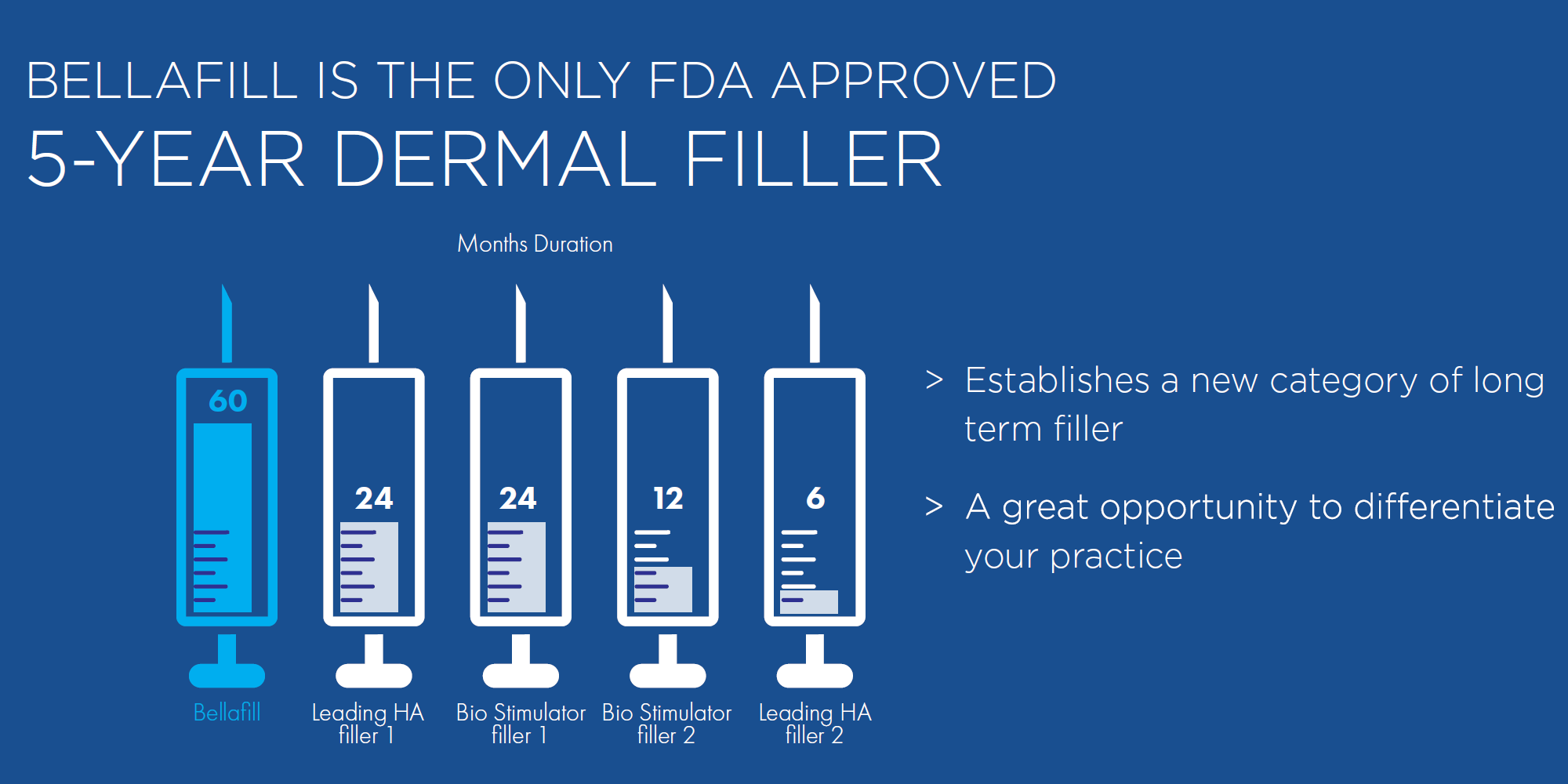
Bellafill is the only FDA approved filler that provides immediate correction and lasts up to 5 years with each treatment. Your patients are demanding a longer lasting alternative to their short acting HA fillers and only Bellafill can best address this need.
- Addresses the #1 Patient Need – 90% of patients are interested in a 5-year dermal filler
- Create Patient Loyalty with Bellafill – 82% of patients would switch providers for a 5-year filler
- Use Bellafill to grow your practice beyond dermal fillers – 88% of patients would spend more money on other aesthetic procedures with the practice that provided them with a 5-year filler
PROVEN SAFE AND EFFECTIVE
Bellafill is the most studied dermal filler on the market. With over 1,542 patients across multiple clinical studies, Bellafill has proven itself time and time again to be safe and effective. In fact, Bellafill’s 12-year Post Market Surveillance data shows a consistently low adverse event (0.12%) rate that is comparable to other HA fillers.

IN A CLINICAL STUDY,
Bellafill was shown to stimulate both Type III and Type I – the ideal types of collagen for natural and long-lasting correction. The natural and long-lasting results are what give Bellafill it’s unprecedented patient satisfaction.
- 87% satisfaction rate in Bellafill’s 5-year clinical study
- Rated the “Most Worth It” by actual patientson RealSelf
MORE ABOUT THE TECHNOLOGY
AND THE OPPORTUNITY
Bellafill increases patient loyalty and helps grow your filler practice and beyond.
Real patients. Real Results.
Hear what other providers are saying about Bellafill.