*Not actual patient
BENEFITS
- Rejuvenates the skin to reduce the signs of aging
- May improve skin smoothness and texture
- Encourages the skin’s natural glow
WHAT YOU CAN EXPECT
- Treatment typically under 30 minutes, depending on the area(s) treated
- Minimal downtime

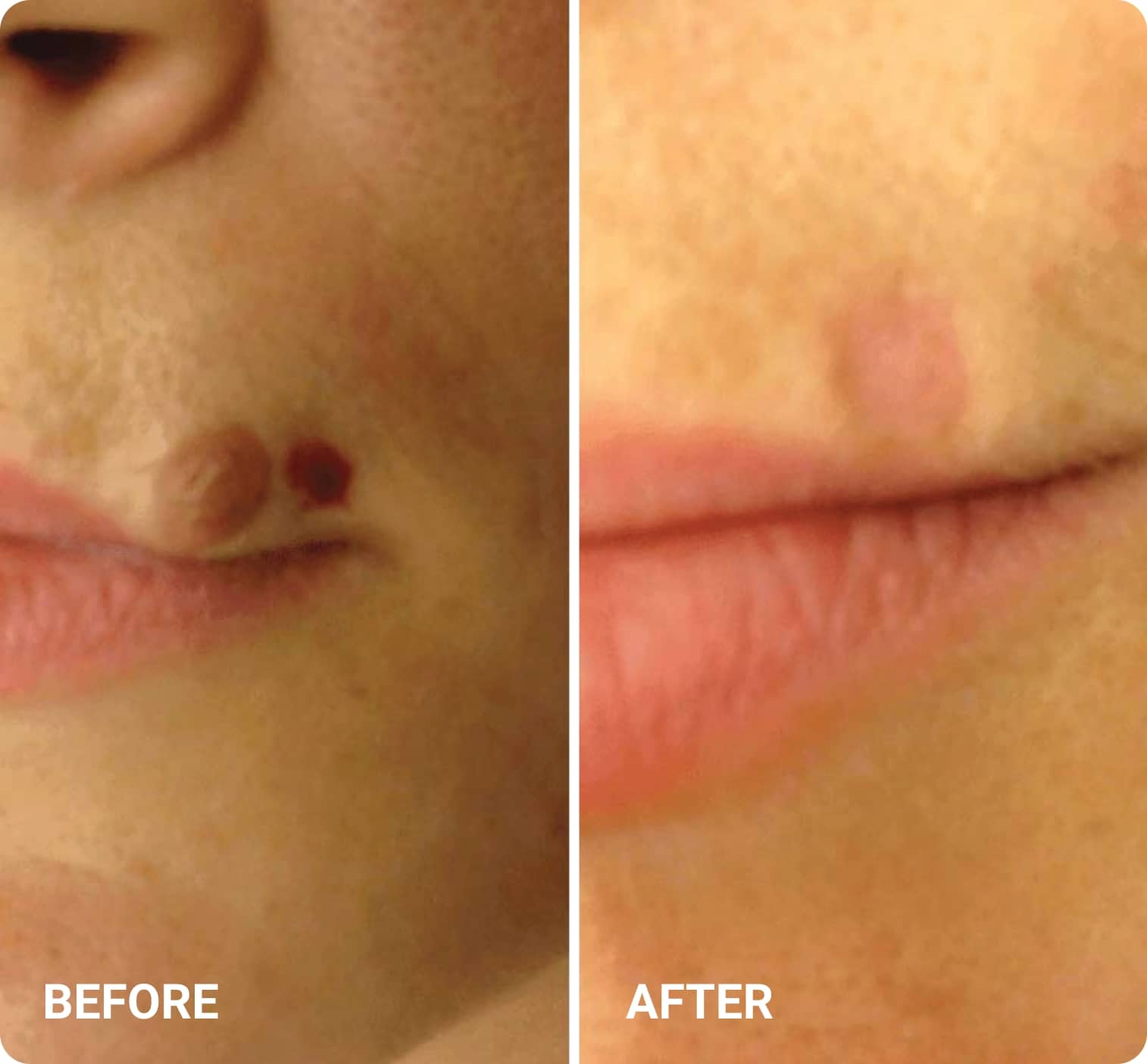
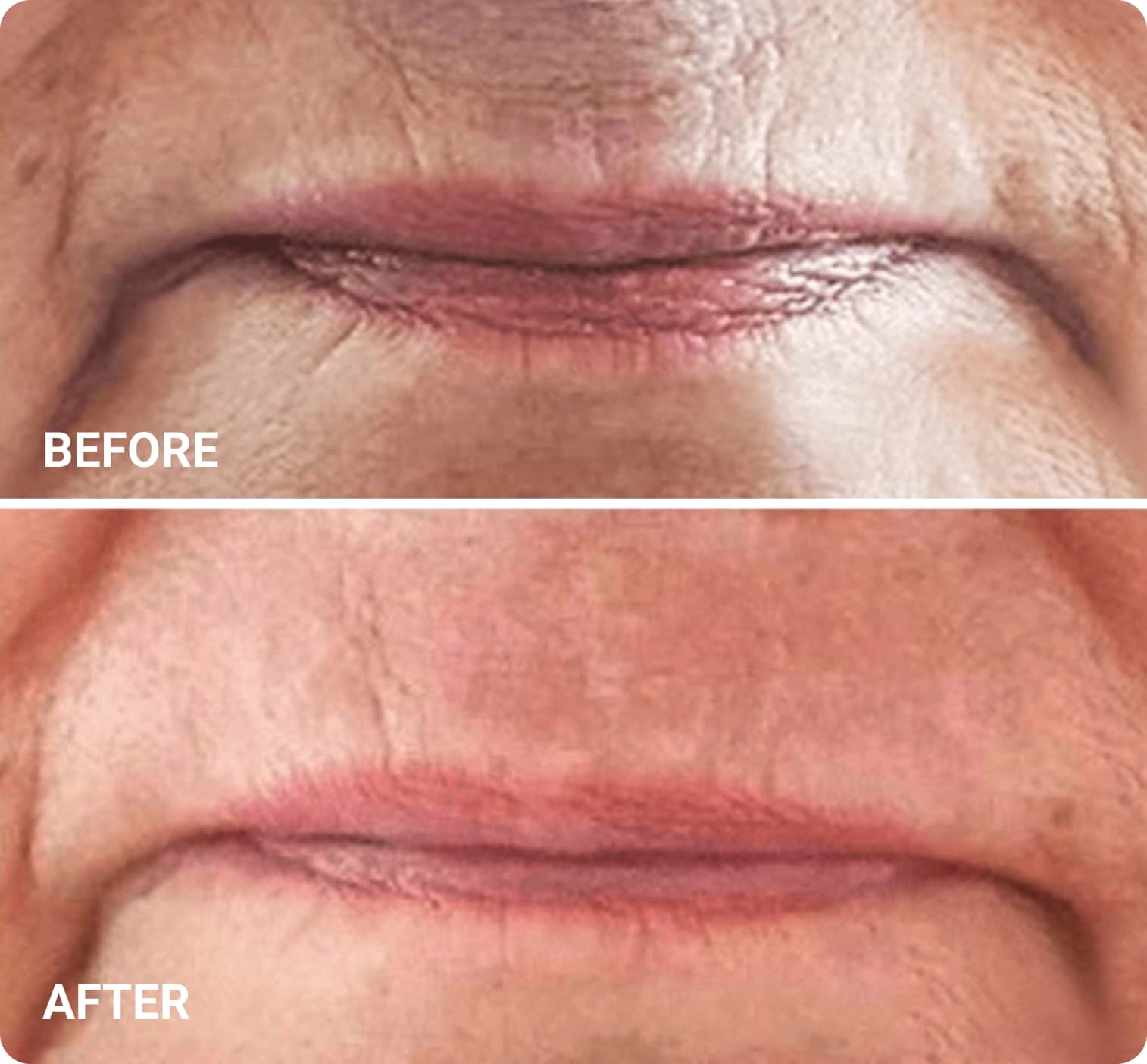
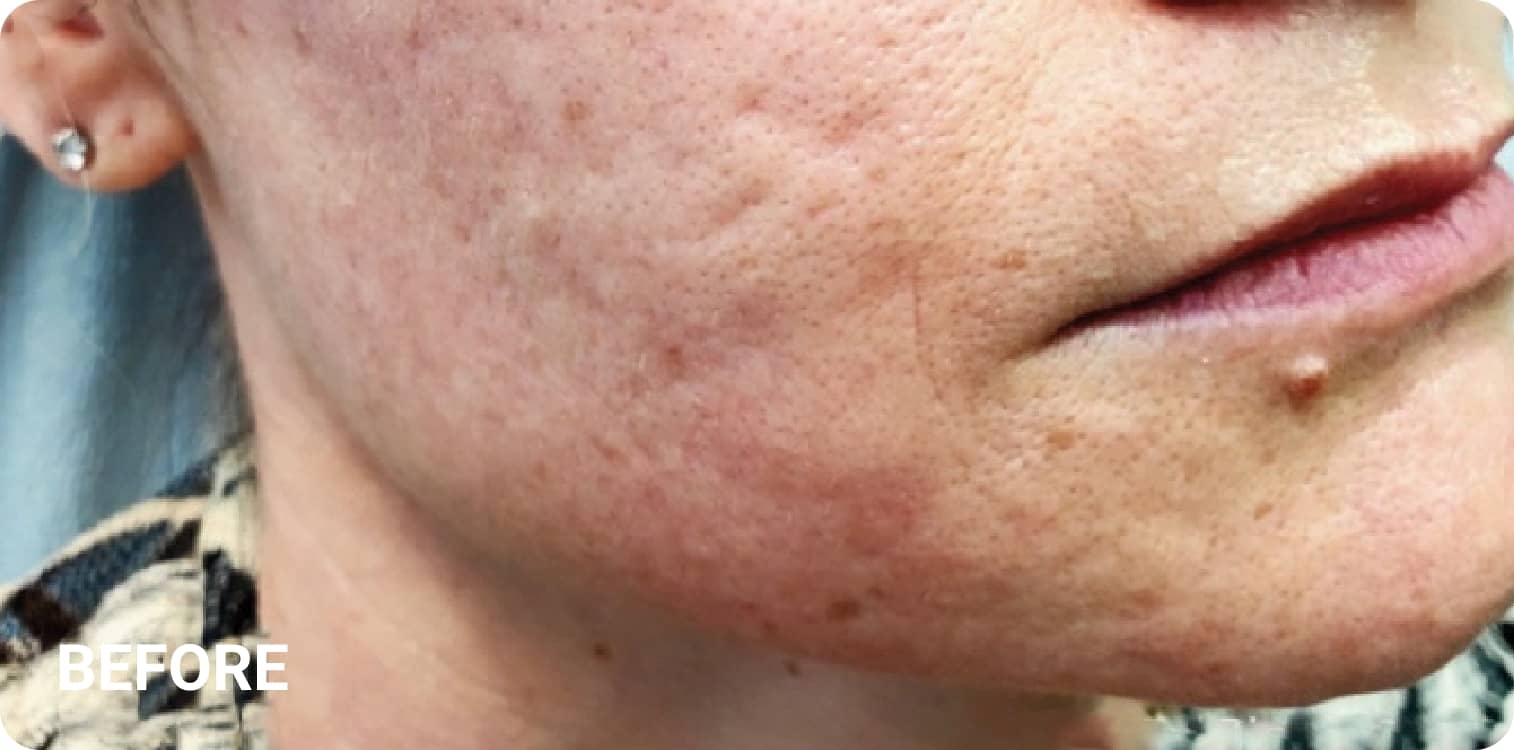
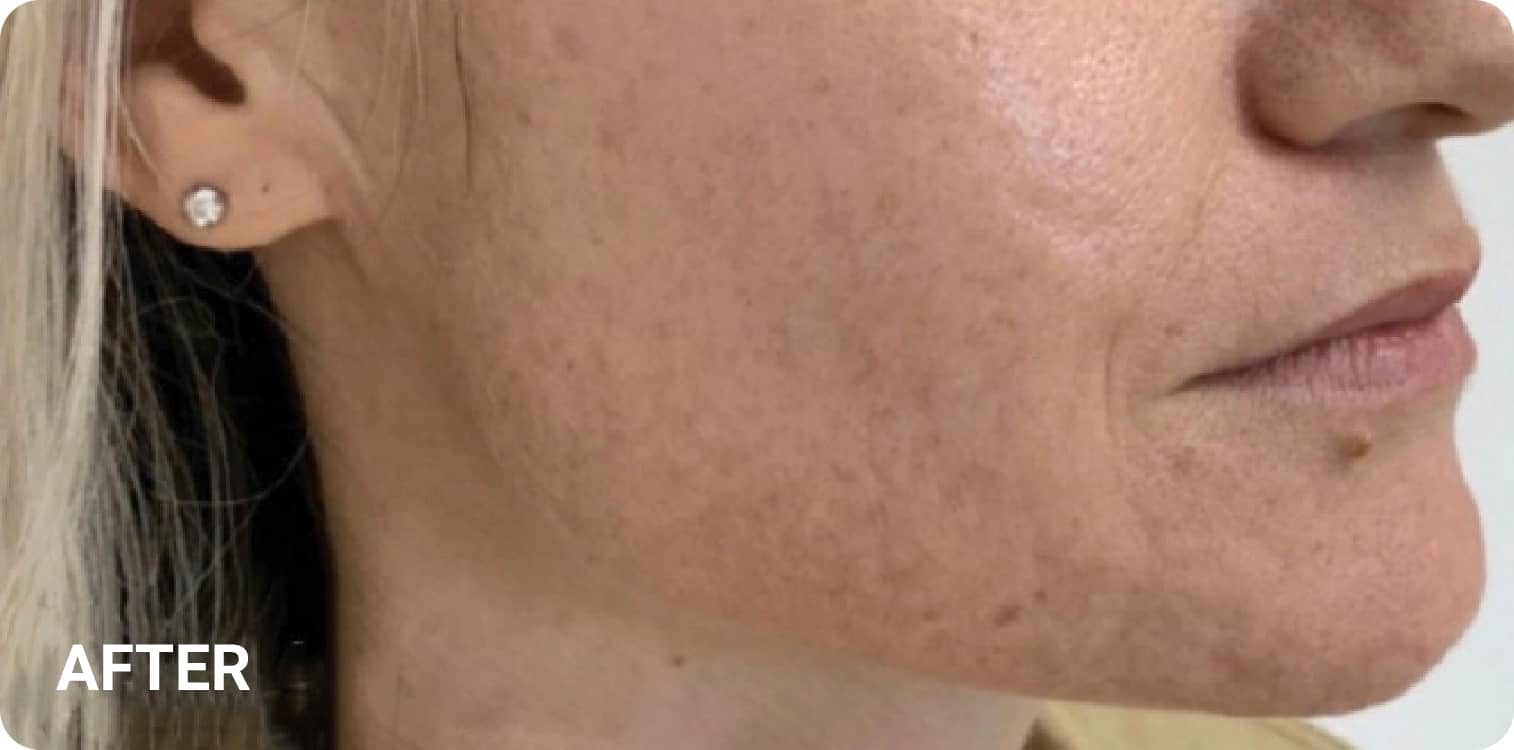
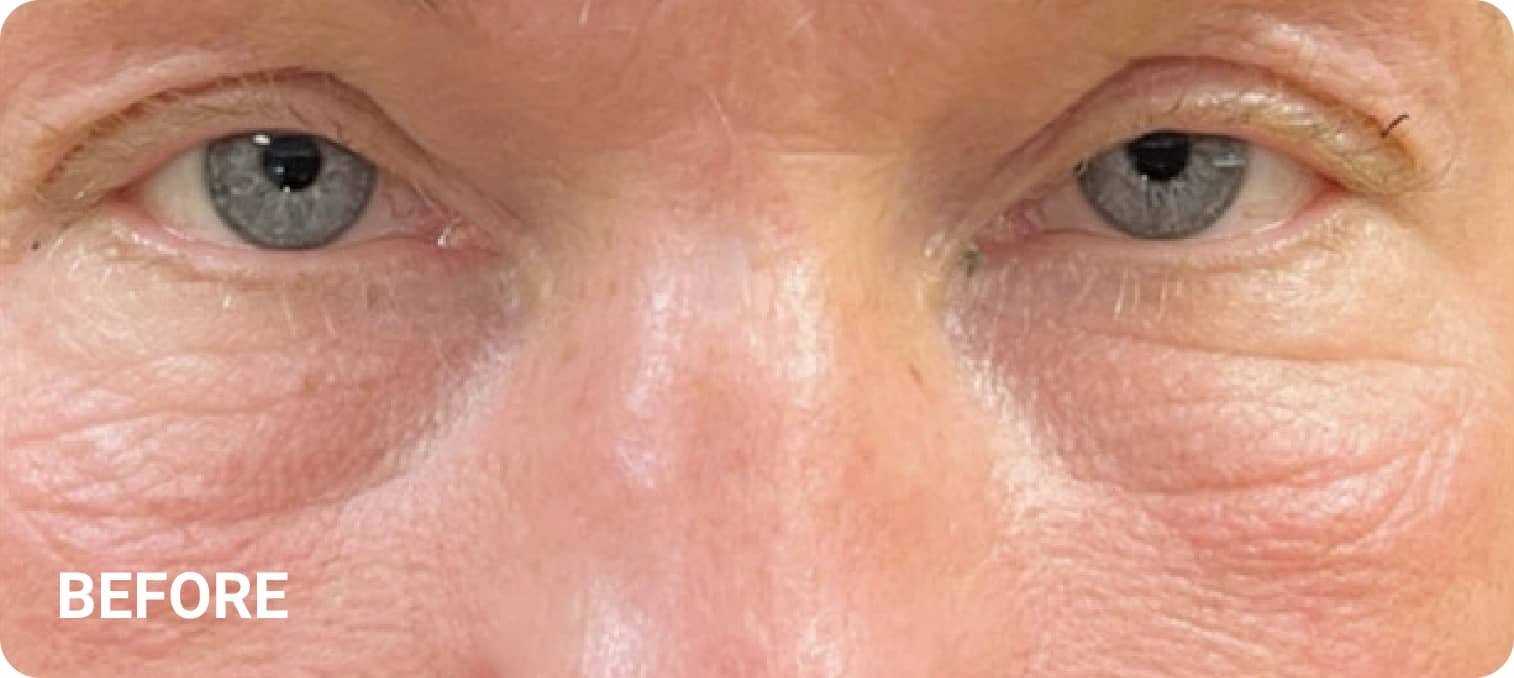
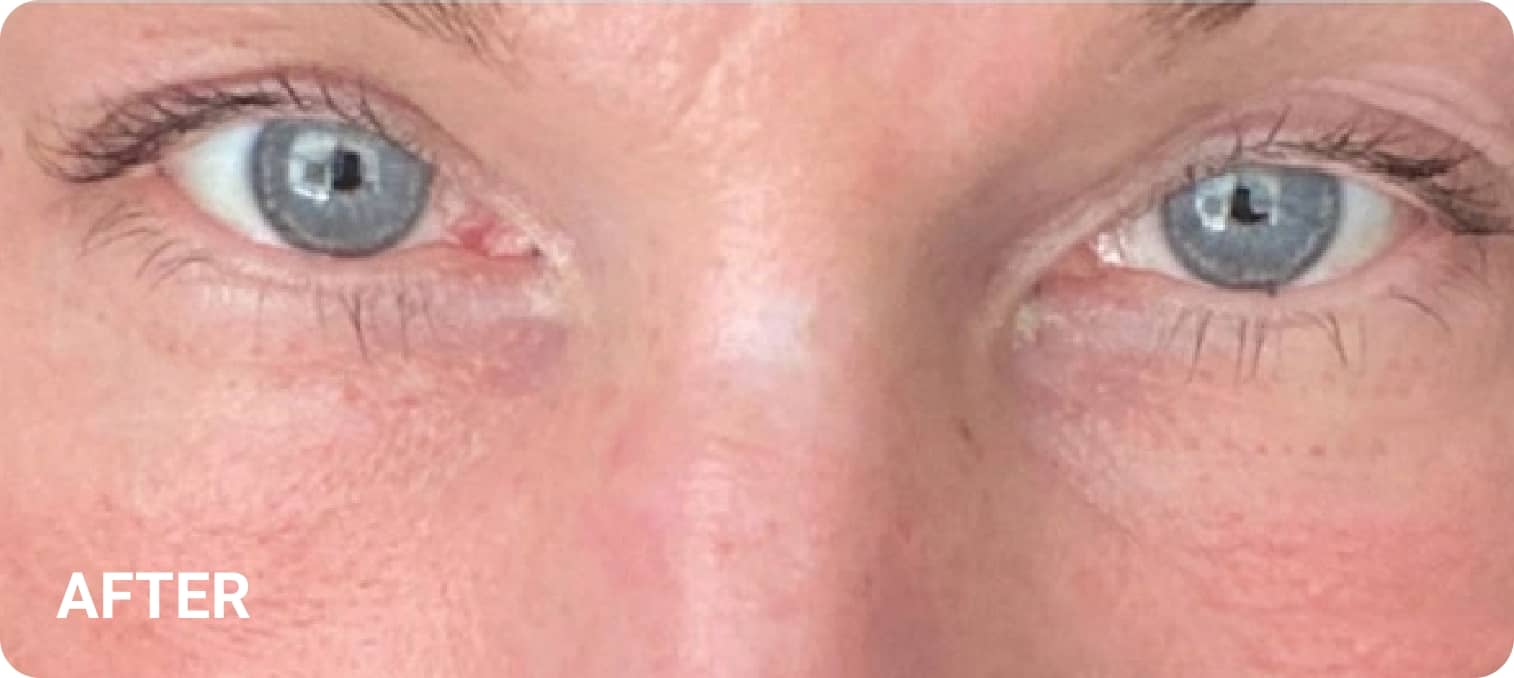
REAL RESULTS
Skin tightening Plasma IQ treatment on a focused facial area, after treatment. Some minor redness is visible.
HOW IT WORKS
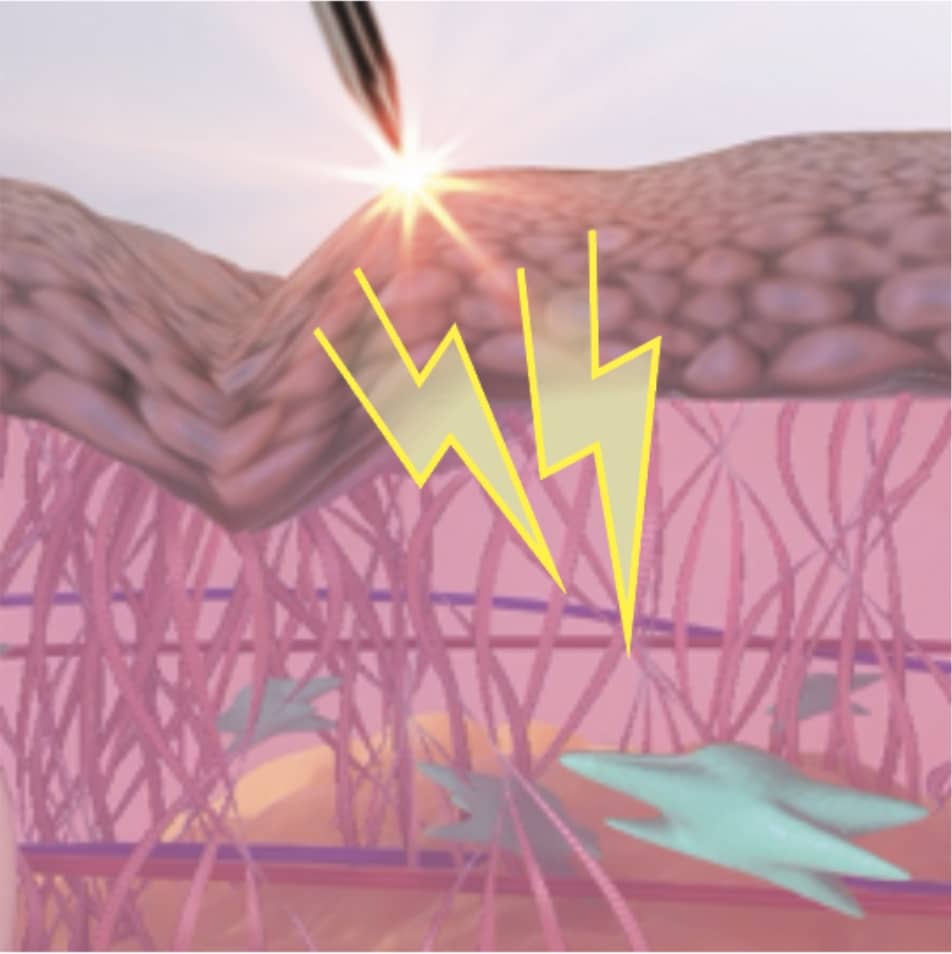
As Plasma IQ approaches the surface of the skin, it ionizes atmospheric nitrogen gas particles in the air to create a plasma arc that looks like a spark.1,2,3
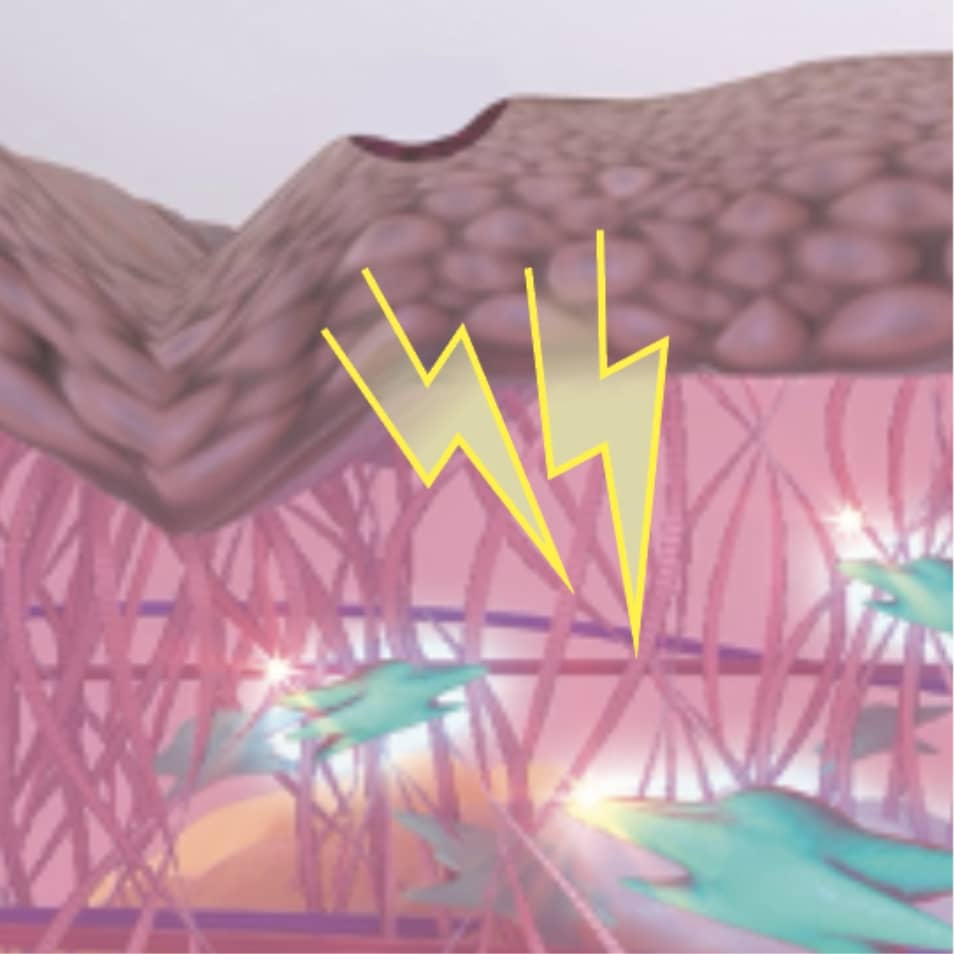
The heat energy delivered by the plasma arc create controlled microinjuries to the upper layers of the skin.3,4,5
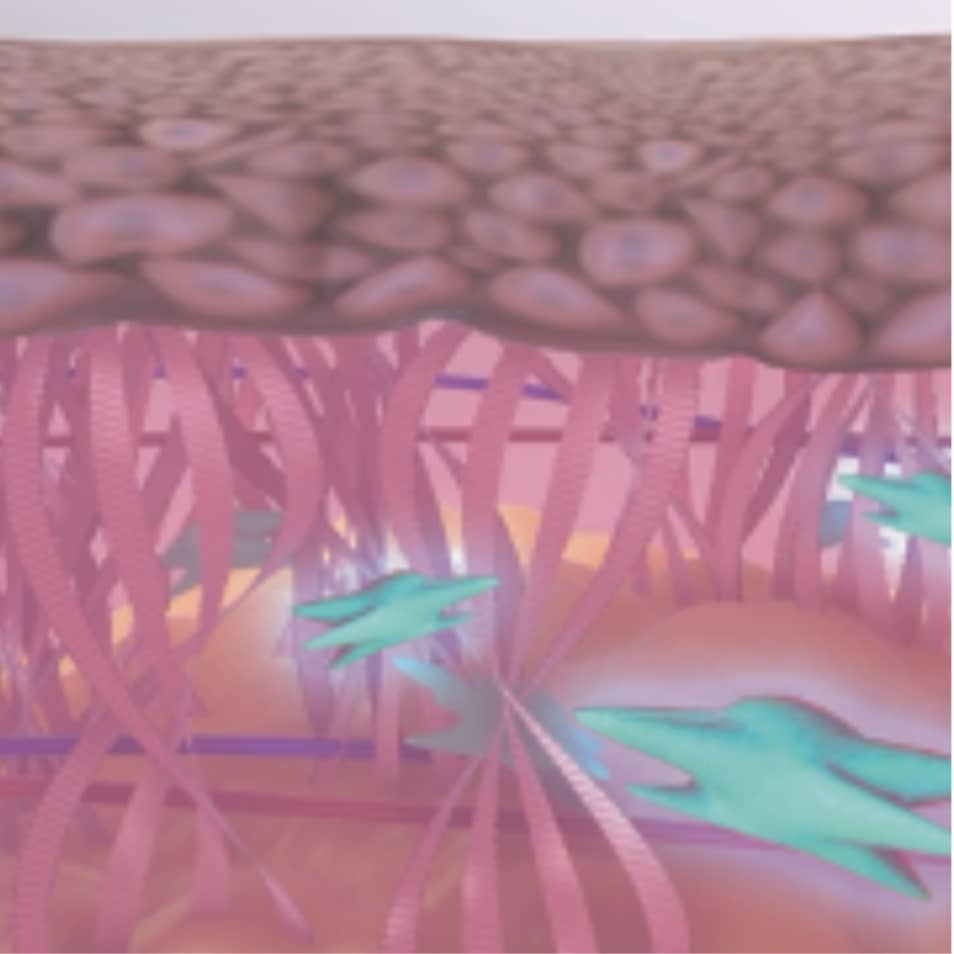
This results in tightening and contraction of the skin to restore skin health and regeneration.3
PLASMA IQ is FDA cleared to be used in the removal and destruction of skin lesions and the coagulation of tissue. The most common side effects are swelling, tenderness, scabbing and redness. PLASMA IQ is Rx only and should only be used by medically licensed and certified practitioners. For full product and safety information, visit https://www.sunevamedical.com/ifu/ ©2020 Neauvia North America.
Loading Providers…

