Give your Silhouette
Instalift® patients
the gift of time
- Bellafill® is the only dermal filler FDA
approved to safely and effectively
treat smile lines for up to 5 years - Try Bellafill and benefit from
SUNEVA regenerative portfolio
price discounts
Add another proven
biostimulator
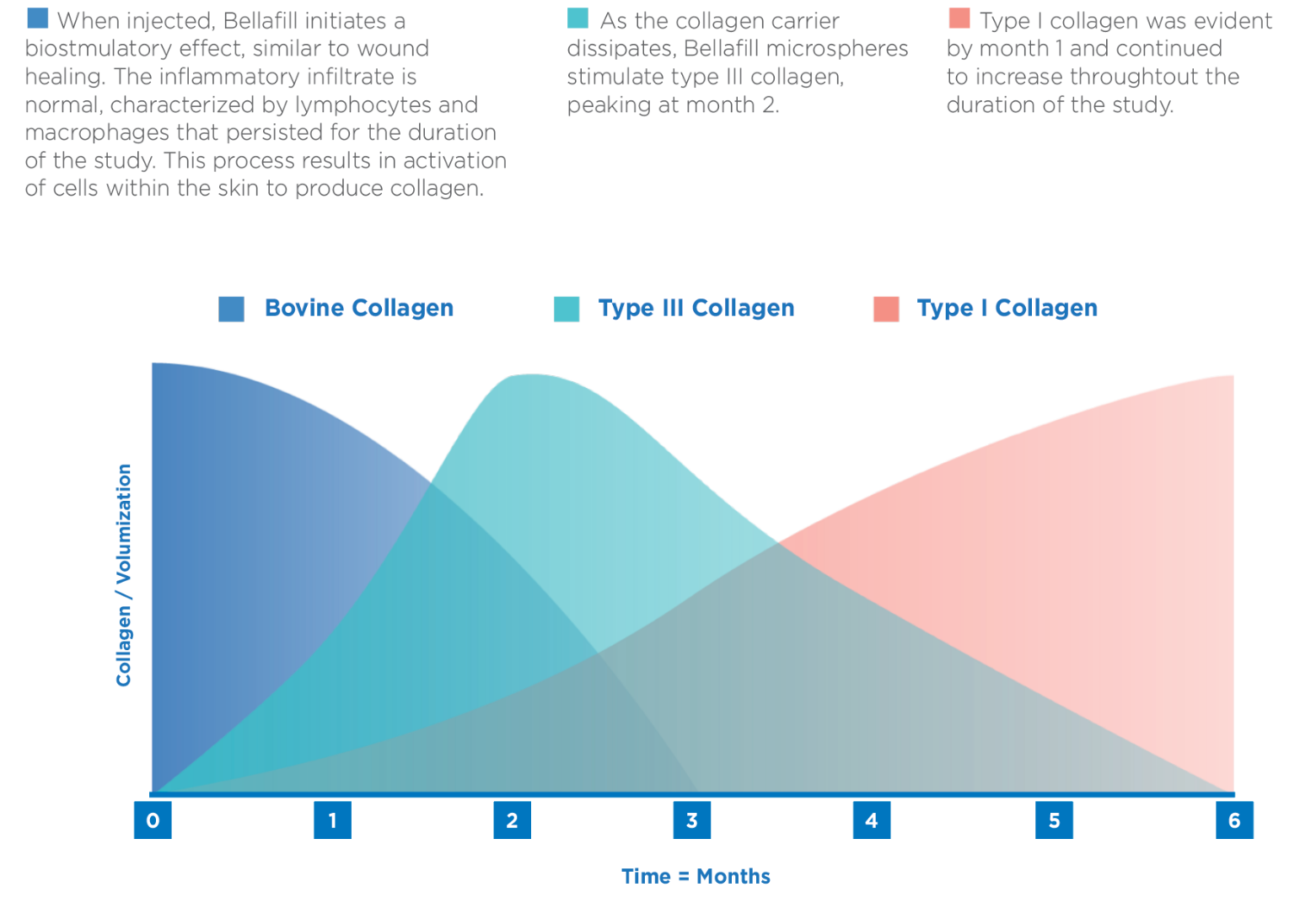
Similar to Silhouette InstaLift, Bellafill has a unique
two-part mechanism of action that delivers immediate
and long-lasting results. Bellafill is the only dermal filler
with microspheres to stimulate collagen for up to 5 years.
Find out how you can save on Bellafill.
Clinically proven safe and effective
MAXIMIZE YOUR ROI
- Add a unique, incremental treatment option for
your Silhouette InstaLift patients - Save on Bellafill purchases with the
SUNEVA Regenerative Portfolio Pricing Program
Find out how you can save on Bellafill.
Data on file, Suneva Medical Inc.
Bellafill®
Bellafill® is indicated for the correction of nasolabial folds and moderate to severe, atrophic, distensible facial acne scars on the cheek in patients over the age of 21 years. Patients who have had a positive reaction to the Bellafill® Skin Test, have a history of severe allergies, have known bovine collagen allergies, are allergic to lidocaine, have bleeding disorders or are prone to thick scar formation and/or excessive scarring should not receive Bellafill®. The safety of Bellafill® for use during pregnancy, breastfeeding, or in patients under 21 has not been established.You may experience temporary swelling, redness, pain, bruising, lumps/bumps, itching, and discoloration at the treatment site. These side effects are usually transient and typically resolve within 1–7 days. You may experience lumps/bumps/papules that may occur more than one month after injection and that may persist. Less common side effects include rash and itching more than 48 hours after treatment, persistent swelling or redness, lumps/bumps, acne, and increased sensitivity at treatment sites. Infrequently, granulomas may occur and may be treated by your licensed physician provider. Be sure to call your licensed provider immediately if you notice any unusual skin reactions around the treatment area. Based on the 5-year Post-Approval Study on nasolabial folds with 1,008 patients, long-term safety of Bellafill® for up to 5 years has been established.
Silhouette Instalift
Silhouette lnstaLift® is indicated for use in mid-face suspension surgery to temporarily fixate the cheek sub-dermis in an elevated position.
IMPORTANT SAFETY CONSIDERATIONS
The Silhouette InstaLift device should not be used in patients with any known allergy or foreign body sensitivities to plastic/ biomaterial or in situations where internal fixation is otherwise contraindicated, (e.g. infection.) The device should also not be used in patients appearing to have very thin soft tissue of the face in which the implant may be visible or palpable.
Like all procedures of this type there is a possibility of adverse events, although not everybody experiences them. These adverse events include but are not limited to infection, minimal acute inflammatory tissue reaction, pain (which may be temporary or persistent in nature), swelling and edema, transient hematoma or bruising and transient rippling or dimple formation. For further safety or product information, please consult your physician.
For more safety information, please consult with your physician and the patient labeling that can be found by visiting our website www.bellafill.com.
Toll-free call (U.S. & Canada): 844-Bellafill (844-235-5234).
Local calls: 858-550-9999. International calls: ++ 858-550-9999.
www.bellafill.com www.sunevamedical.com
SM 3055 Rev00